In this article, You are going to read the composition and structure of the atmosphere – for Geography UPSC.
What is the Atmosphere?
- The envelope of gases surrounding the earth is called the atmosphere. It forms a protective boundary between the outer space and the biosphere.
- The atmosphere is a dynamic collection of gases that constantly move and change. These gases form several layers around Earth that are loosely defined by composition and temperature.
- The gases of the present atmosphere are not the direct residue of the early stage of earth’s formation. They are a product of progress through volcanic eruptions, hot springs, chemical breakdowns of solid matter and redistribution from the biosphere.
- The atmosphere is a significant component of the biospheric ecosystem because life on the earth’s surface is because of this atmosphere otherwise the earth would have become barren like the moon.
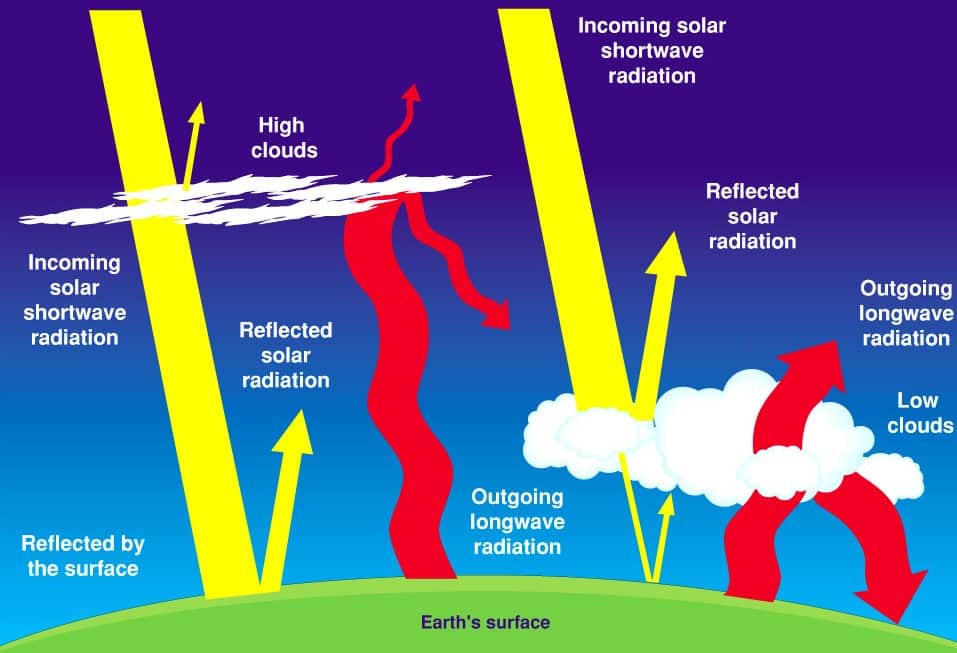
- It protects the earth from the harmful radiation from the sun. It acts as a greenhouse by allowing short-wave radiation (from Sun) and trapping long-wave terrestrial radiation (from Earth’s surface).
- All life forms need a particular range of temperature and a specific range of frequencies of solar radiation to carry out their biophysical processes. The atmosphere absorbs certain frequencies and lets through some other frequencies of solar radiation. In other words, the atmosphere regulates the entry of solar radiation.
- The atmosphere also keeps the temperature over the earth’s surface within certain limits. In the absence of the atmosphere extremes of temperature would exist between day and night over the earth’s surface.
- The atmosphere also takes care of extra-terrestrial objects like meteors that get burnt up while passing through the atmosphere (mesosphere to be precise) due to friction.
Composition of the atmosphere
- The gases in the atmosphere are composed of neutral, uncharged particles.
- Except for the noble gases, atoms in the gas phase share electrons with other atoms in chemical bonds so that their electron count can approach the more stable filled-shell configuration.
- The Earth’s atmosphere consists of a mixture of noble gas atoms and many kinds of molecules.
- The atmosphere is composed of –
- Gases
- Vapour
- Particulates
- In addition, it contains huge numbers of solid and liquid particles, collectively called aerosols.
Gases
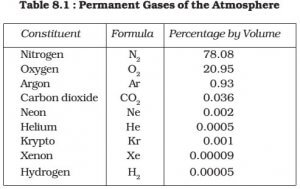
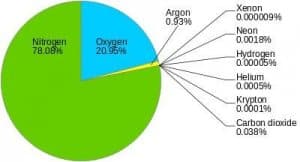
- Nitrogen and oxygen make up nearly 99% of the clean, dry air. The remaining gases are mostly inert and constitute about 1% of the atmosphere.
- Oxygen, although constituting only 21% of the total volume of the atmosphere, is the most important component among gases. All living organisms inhale oxygen. Besides, oxygen can combine with other elements to form important compounds, such as oxides. Also, combustion is not possible without oxygen.
- Nitrogen accounts for 78% of total atmospheric volume. It is a relatively inert gas and is an important constituent of all organic compounds. The main function of nitrogen is to control combustion by diluting oxygen. It also indirectly helps in the oxidation of different kinds.
- Carbon Dioxide which constitutes only about 0.038% of the dry air and is a product of combustion. Green plants, through photosynthesis, absorb carbon dioxide from the atmosphere and use it to manufacture food and keep other biophysical processes going.
- Being an efficient absorber of heat, carbon dioxide is considered to be of great climatic significance. Carbon dioxide is considered to be a very important factor in the heat energy budget.
- With the increased burning of fossil fuels – oil, coal, and natural gas – the carbon dioxide percentage in the atmosphere has been increasing at an alarming rate.
- More carbon dioxide in the atmosphere means more heat absorption. This could significantly raise the temperature at lower levels of the atmosphere thus inducing drastic climatic changes.
- Carbon dioxide and water vapour are found only up to 90 km from the surface of the earth.
- The third important gas is Argon which constitutes only about 0.93%.
- Ozone (03) is another important gas in the atmosphere, which is actually a type of oxygen molecule consisting of three, instead of two, atoms. It forms less than 0.00006% by volume of the atmosphere and is unevenly distributed. It is between 20 km and 25 km altitude that the greatest concentrations of ozone are found. It is formed at higher altitudes and transported downwards.
- Ozone plays a crucial role in blocking the harmful ultraviolet radiation from the sun.
- Other gases found in almost negligible quantities in the atmosphere are neon, helium, hydrogen, xenon, krypton, methane, etc.
Water Vapour
- The vapour content in the atmosphere ranges from 0 to 5 % by volume.
- The atmospheric vapour is received through the evaporation of moisture and water from the water bodies (like seas and oceans, lakes, tanks and ponds, rivers, etc.), vegetation, and soil cover.
- Vapour depends on temperature and therefore it decreases from the equator poleward in response to decreasing temperature towards the poles.
- The content of the vapour in the surface air in the moist tropical areas, at 50-degree and 70-degree latitudes, is 2.6%, 0.9%, and 0.2% (by volume) respectively.
- The content of vapour decreases upward.
- More than 90% of the total atmospheric vapour is found up to the height of 5 km.
- The moisture content in the atmosphere creates several forms of condensation and precipitation e.g. clouds, fogs, dew, rainfall, frost, hailstorm, ice, snowfall, etc.
- Vapour is almost transparent for incoming shortwave solar radiation so that the electromagnetic radiation waves reach the earth’s surface without many obstacles but vapour is less transparent for outgoing longwave terrestrial radiation and therefore it helps in heating the earth’s surface and lower portion of the atmosphere because it absorbs terrestrial radiation.
Particulate Matter
- The Solid Particles present in the atmosphere consist of sand particles (from weathered rocks and also derived from volcanic ash), pollen grains, small organisms, soot, ocean salts; the upper layers of the atmosphere may even have fragments of meteors which got burnt up in the atmosphere.
- These particulates help in the absorbing, reflecting, and scattering of the solar radiation which adds the varied charming colour of red and orange at sunrise and sunset.
- The sky appears blue in colour due to the selective scattering of solar radiation by dust particles.
- Salt particles become hygroscopic nuclei and thus help in the formation of water drops, clouds, and various forms of condensation and precipitation.
- Hygroscopic nucleus – a microscopic particle (e.g. of sulphur dioxide, salt, dust, or smoke) in the free air, on to which water vapor may condense to form droplets.
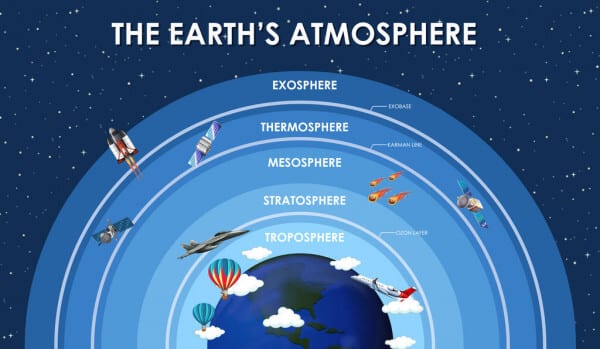
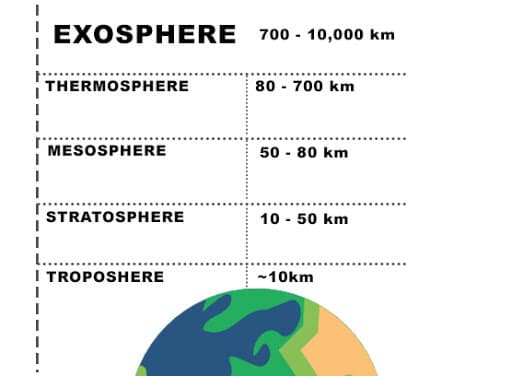
Structure of the Atmosphere
The atmosphere can be divided into different layers according to composition, density, pressure, and temperature variations.
Based on Composition:
According to its composition, broadly it is divided into two layers-
- homosphere
- heterosphere
Homosphere
- The homosphere is the lower segment of the two-part division of atmosphere and further consists of three regions namely troposphere, stratosphere and mesosphere.
- The Troposphere is the earth’s weather layer. It contains nearly all weather conditions. As you go up in altitude the temperature goes down. It is the bottom-most layer of the
- The Stratosphere is the middle region of the Homosphere.
- The Mesosphere is the top layer of the Homosphere.
- All the three regions have the same composition of air. However, the concentration of air keeps decreasing significantly as the altitude increases.
- It extends from the earth’s surface up to an altitude of 80km.
Heterosphere
- The heterosphere is the layer of an atmosphere where the gases are separated out by molecular diffusion with increasing altitude such that lighter species become more abundant relative to heavier species.
- In the Heterosphere, there are two regions: The Thermosphere and the Exosphere. These two regions are considered outer space.
- The thermosphere is the bottom region of the Heterosphere.
- The exosphere is the top region of the Heterosphere.
- It begins over 80km and extends up to 10,000 km.
Based on Change in temperature
The atmosphere can be divided into five layers according to the diversity of temperature and density. They are:
- Troposphere
- Stratosphere
- Mesosphere
- Thermosphere (Ionosphere)
- Exosphere
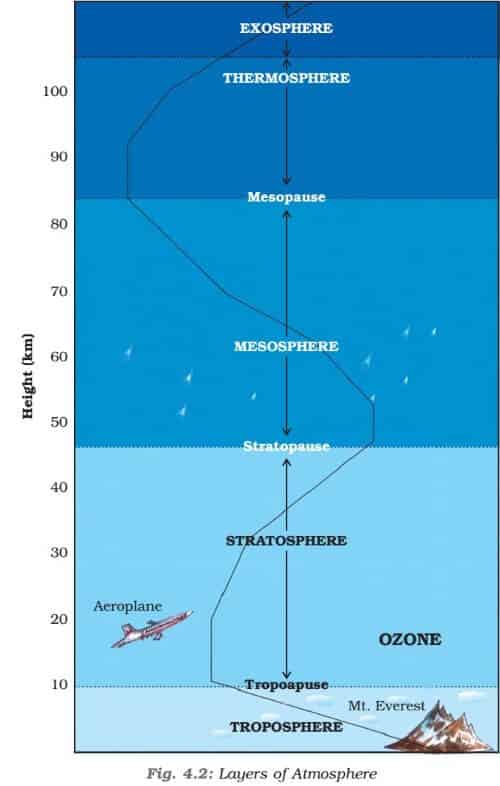
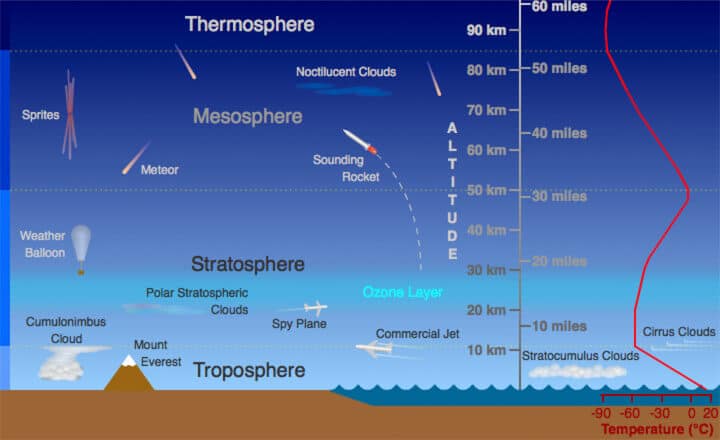
Troposphere:
- The troposphere is the lowest layer of Earth’s atmosphere. It extends up to 18km at the equator, 13 km at mid-latitude and about 8km at poles.
- Most of the mass (about 75-80%) of the atmosphere is in the troposphere.
- The thickness is greater at the equator, because the heated air rises to greater heights.
- The troposphere ends with the Tropopause.
- The temperature in this layer, as one goes upwards, falls at the rate of 5°C per kilometer, and reaches -45°C at the poles and -80°C over the equator at Tropopause (greater fall in temperature above equator is because of the greater thickness of troposphere – 18 km).
- The fall in temperature is called ‘lapse rate’. (more about this in future posts)
- The troposphere is marked by temperature inversion, turbulence and eddies.
- It is also meteorologically the most significant zone in the entire atmosphere (Almost all the weather phenomena like rainfall, fog and hailstorm etc. are confined to this layer).
- It is also called the convective region, since all convection stops at Tropopause.
- The troposphere is the theatre for weather because all cyclones, anticyclones, storms and precipitation occur here, as all water vapours and solid particles lie within this.
- The troposphere is influenced by seasons and jet streams.
Tropopause
- The tropopause is the atmospheric boundary that demarcates the troposphere from the stratospherere.
- This layer is marked by constant temperatures.
Stratosphere:
- It lies above the troposphere and extends uniformly across the globe up to 50km.
- The temperature in this layer remains constant for some distance but then rises to reach a level of 0°C at 50 km altitude.
- This rise is due to the presence of ozone (harmful ultraviolet radiation is absorbed by ozone).
- This layer is almost free from clouds and associated weather phenomenon, making conditions most ideal for flying aeroplanes. So aeroplanes fly in lower stratosphere, sometimes in upper troposphere where weather is calm.
- Sometimes, cirrus clouds are present at lower levels in this layer.
Mesosphere:
- The mesosphere extends from 50 – 80 km.
- The temperature again decreases in this layer and reaches its minimum mark averaging -90 C. Although this temperature can vary.
- The homogenous layer extends up to the mesosphere.
- At the upper boundary of the mesosphere, there exists a layer of ions extending in the other layer.
- This layer of ions or charged particles is helpful in reflecting the radio waves and helps in telecommunication.
Thermosphere:
- In thermosphere temperature rises very rapidly with increasing height.
- Ionosphere is a part of this layer. It extends between 80-400 km.
- This layer helps in radio transmission. In fact, radio waves transmitted from the earth are reflected back to the earth by this layer.
- Person would not feel warm because of the thermosphere’s extremely low pressure.
- The International Space Station and satellites orbit in this layer. (Though temperature is high, the atmosphere is extremely rarified – gas molecules are spaced hundreds of kilometers apart. Hence a person or an object in this layer doesn’t feel the heat)
- Aurora’s are observed in lower parts of this layer.
Ionosphere:
- This layer is located between 80 km and 400 km and is an electrically charged layer.
- It lies from the upper mesosphere to the thermosphere.
- The charged particles are ionized by absorption of cosmic rays, gamma rays, X-rays and shorter wavelengths of ultraviolet rays.
- It is in this layer that incoming space vehicles and meteorites begin to heat due to friction.
- Temperature again starts increasing with height because of radiation from the sun.
Exosphere
- This is the uppermost layer of the atmosphere extending beyond the ionosphere above a height of about 400 km.
- The air is extremely rarefied and the temperature gradually increases through the layer.
- Light gases like helium and hydrogen float into the space from here.
- Temperature gradually increases through the layer. (As it is exposed to direct sunlight)
- This layer coincides with space.
Quiz:
- Jet aircraft fly in the stratosphere, not in the troposphere.
- The stratosphere is the layer with auroras, not the troposphere.
- Most of the Weather patterns occur in the troposphere, not in the stratosphere.
Select the correct answer code:
a) 1, 2
b) 2, 3
c) 1, 3
d) 1, 2, 3
Solution: c)
Many jet aircraft fly in the stratosphere because it is very stable.
Auroras are the result of disturbances in the magnetosphere caused by the solar wind. These particles, mainly electrons, and protons precipitate into the upper atmosphere (thermosphere/exosphere).
The troposphere is the lowest layer of Earth’s atmosphere and is also where nearly all weather conditions take place.
- In the stratosphere, temperature increases with altitude.
- In the mesosphere, temperature decreases with altitude.
- The lowest temperature of the atmosphere is recorded in the upper part of the mesosphere.
- The tropopause is an isothermal zone.
Select the correct answer code:
a) 1, 2
b) 1, 2, 3
c) 3, 4
d) 1, 2, 3, 4
Solution: d)
The top of the mesosphere, called the mesopause, is the coldest part of Earth’s atmosphere. Temperatures
in the upper mesosphere fall as low as −101 °C (172 K; −150 °F), varying according to latitude and season.
An isothermal layer is defined as a vertical column of air having a constant temperature with height.
- 99% of earth’s atmosphere is confined to the height of 320 km from earth’s surface.
- Hydrogen and Oxygen were the major constituents of early atmosphere.
Which of the above statements is/are incorrect?
- a) 1 only
- b) 2 only
- c) Both 1 and 2
- d) Neither 1 nor 2
Solution: c)
The air is an integral part of the earth’s mass and 99 percent of the total mass of the atmosphere is confined to the height of 32 km from the earth’s surface.
Oxygen was absent in the early atmosphere. Atmospheric levels of oxygen did not rise until oxygenic photosynthesis was well established.

Hi
Carbon dioxide present up to 9 Km I think, correct me if am wrong.
It will be 90 km, refer to NCERT Chapter 8 “COMPOSITION AND STRUCTURE
OF ATMOSPHERE”.
https://ncert.nic.in/ncerts/l/kegy208.pdf
thank you so much lotus arise team
Thank you
Thanx so much for this wonderful explanation and notes
Thanks for the notes admin..
sir isn’t the normal lapse rate 6.5 in troposphere?