- Genetic engineering (also called genetic modification) is a process that uses laboratory-based technologies to alter the DNA makeup of an organism. This may involve changing a single base pair (A-T or C-G), deleting a region of DNA, or adding a new segment of DNA.
- Gene Editing is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living organism. Unlike early genetic engineering techniques that randomly insert genetic material into a host genome, genome editing targets the insertions to site-specific locations.
- CRISPR is widely considered the most precise, most cost-effective, and quickest way to edit genes.
- The techniques of genetic engineering which include creation of recombinant DNA, use of gene cloning and gene transfer, overcome this limitation and allows us to isolate and introduce only one or a set of desirable genes without introducing undesirable genes into the target organism.
- There are three basic steps in genetically modifying an organism —
- identification of DNA with desirable genes;
- introduction of the identified DNA into the host;
- maintenance of introduced DNA in the host and transfer of the DNA to its progeny.
Techniques of Genetic Engineering
- DNA/RNA extraction: The DNA/RNA is isolated and extraction from cells, this can be done by breaking open the cells using enzymes to destroy macromolecules that are not needed.
- PCR (Polymerase Chain Reaction): The techniques that amplify a single segment of DNA into a thousand copies within a short period. Desired DNA amplified through recurrent replication process.
- Enzymes: Restriction Endonucleases, DNA Ligase, DNA Polymerase.
- Gel Electrophoresis: Gel electrophoresis is the technique that separates molecules according to their size using charge in the electric field.
- Hybridization, Southern and Northern Blotting
- Molecular Cloning
- Three T’s: Transduction, Transfection, Transformation
Benefits of genetic engineering
- Genetic modification is a faster and more efficient way of getting the same results as selective breeding.
- Improve crop yields or crop quality, which is important in developing countries. This may help reduce hunger around the world.
- Introduce herbicide resistance, which results in less herbicides being used, as weeds are quickly and selectively killed.
- Insect and pest resistance can be developed and inserted into the plants. The plant produces toxins, which would discourage insects from eating the crop.
- Sterile insects could be created such as a mosquito. They would breed, which would lead to infertile offspring. This may help with spread of diseases, such as malaria, dengue fever and the Zika virus.
Risks of genetic engineering
- Transfer of the selected gene into other species. What benefits one plant may harm another.
- Some people believe it is not ethical to interfere with nature in this way. Also, GM crop seeds are often more expensive and so people in developing countries cannot afford them.
- GM crops could be harmful, for example toxins from the crops have been detected in some people’s blood.
- GM crops could cause allergic reactions in people.
- Pollen produced by the plants could be toxic and harm insects that transfer it between plants.
Difference between Gene therapy and Gene editing
- All concepts of Gene therapy, Gene editing, and CRISPR CAS9 are interlinked.
- We use Gene editing for multiple reasons like designer babies, treatment of genetic disorders, for invention of medicines, etc, if we are editing Gene for health-related then its called Gene therapy. Besides, there is also a difference of degree, in Gene therapy we don’t replace the Gene.
- In gene editing, a mutated gene is revised, removed, or replaced at the DNA level.
- In gene therapy, the effect of a mutation is offset by inserting a “healthy” version of the gene, and the disease-related genes remain in the genome.
- Both approaches may provide a durable benefit to patients, and both gene therapy and gene editing, alone or in combination, may lend themselves to the development of transformative genomic medicines.
- Gene therapy is a technique that modifies a person’s genes to treat or cure a disease. Gene therapies can work by several mechanisms:
- Replacing a disease-causing gene with a healthy copy of the gene
- Inactivating a disease-causing gene that is not functioning properly
- Introducing a new or modified gene into the body to help treat a disease
- Gene therapy products are being studied to treat diseases including cancer, genetic diseases, and infectious diseases.
- Types
- Somatic Gene Therapy: Effects will not be transferred to next generation
- Germline Gene Therapy: Effects transferred to next generation
- Types
Mitochondrial Gene Therapy (MGT)
- Mitochondria are tiny rod-like structures in cells that act as powerhouses, generating the energy that allows our bodies to function. Unusually, they have their own DNA, distinct from the genetic material within the cell nucleus.
- Mitochondrial DNA (mtDNA) makes up about 0.1% of a cell’s total DNA and does not affect individual characteristics such as appearance and personality.
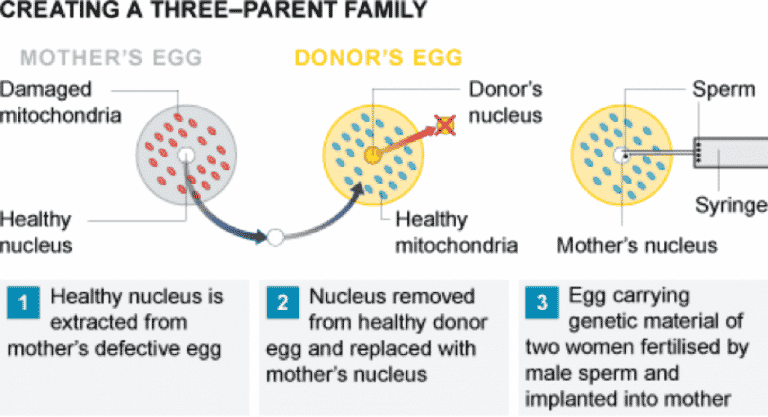
- MGT techniques essentially swap a woman’s defective mitochondrial DNA with that of a donor. The resulting embryo’s DNA will mostly come from the two parents who supplied the egg and sperm, but a tiny proportion – a fraction of a percentage – will come from the donor.
- All cells have mitochondria, which are like power packs for the cells and create the energy that keeps cells alive. While a child’s DNA is a mixture from both the mother and father, mitochondria are separate “packages of genetics” that come solely from the mother.
- Some people have a mitochondrial disease — a problem with the genetics in their mitochondria — which can lead to severe, life-threatening conditions, although this is rare. One treatment for a woman who might have one of these diseases is to replace the mitochondria in her eggs via IVF. This can be done via a process like the one used in Greece where the DNA is taken out of the woman’s egg and put into a donor woman’s egg once the DNA has been stripped from it, which is then fertilized with sperm to create an embryo.
Why is it so controversial?
- Some people don’t like the idea of a baby having three biological parents, and argue that mitochondrial DNA goes some way to shaping important characteristics, such as personality. But the scientific consensus is that swapping mitochondria is similar to changing a battery – it’s unlikely to have much, if any, influence over a person’s behaviour.
- Others have argued that the technique is unnecessary. After all, it won’t help those who have already been born with mitochondrial diseases. Parents often don’t find out they are carriers of these diseases until they give birth to sick children. And those who do know they could pass on a disease have other options, such as using a donor egg. The technique is specifically for people who carry genes for the disease, but want to have a child genetically related to them.
- Another concern is that, by creating a new mix of genetic material, embryologists are creating lasting genetic changes that will be passed down through generations, before we have a chance to find out if they are dangerous. Some argue that this starts us on a slippery slope of germ-line editing – one that could eventually lead to “designer babies”.
Mitochondrial Diseases
- Mitochondrial diseases are a group of disorders caused by dysfunctional mitochondria. Mitochondria are found in every cell of the human body except red blood cells.
- Mitochondrial disorders may be caused by mutations (acquired or inherited), in mitochondrial DNA (mtDNA), or in nuclear genes that code for mitochondrial components.
- They may also be the result of acquired mitochondrial dysfunction due to adverse effects of drugs, infections, or other environmental causes.
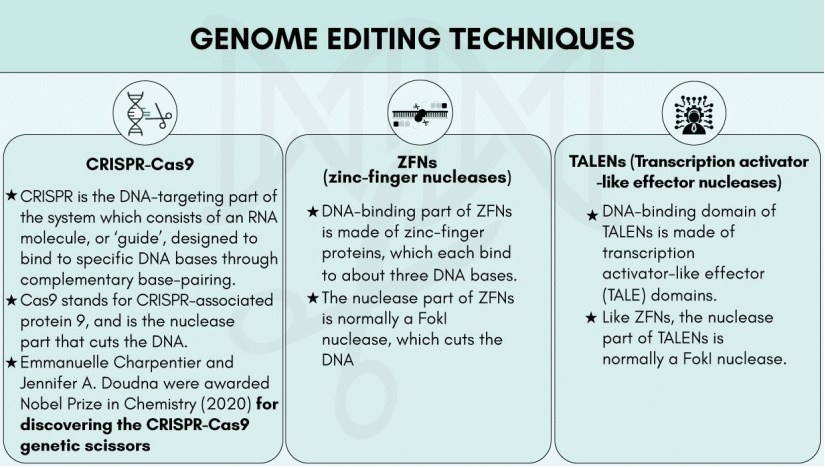
CRISPR-Cas9
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) – Cas9 (CRISPR-associated protein 9) is a unique technology that enables geneticists and medical researchers to edit parts of the genome by removing, adding, or altering sections of the DNA.
It is currently the simplest, most versatile, and precise method of genetic manipulation and is therefore causing a buzz in the science world.
How does it work?
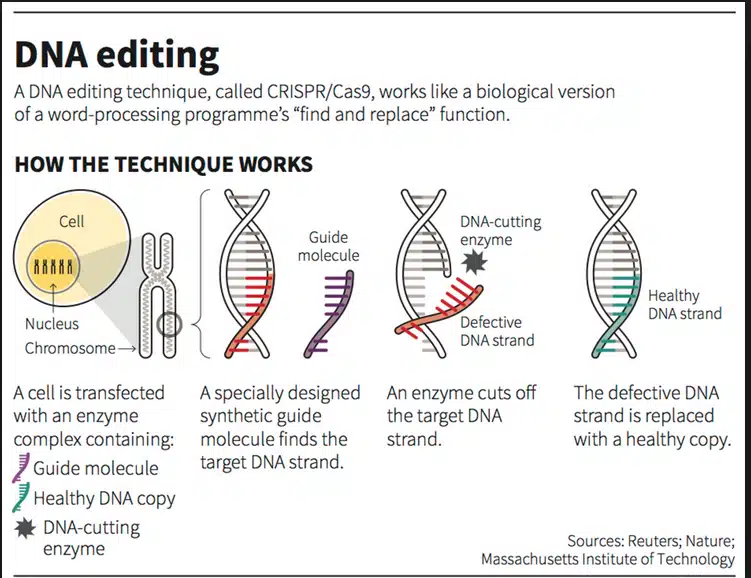
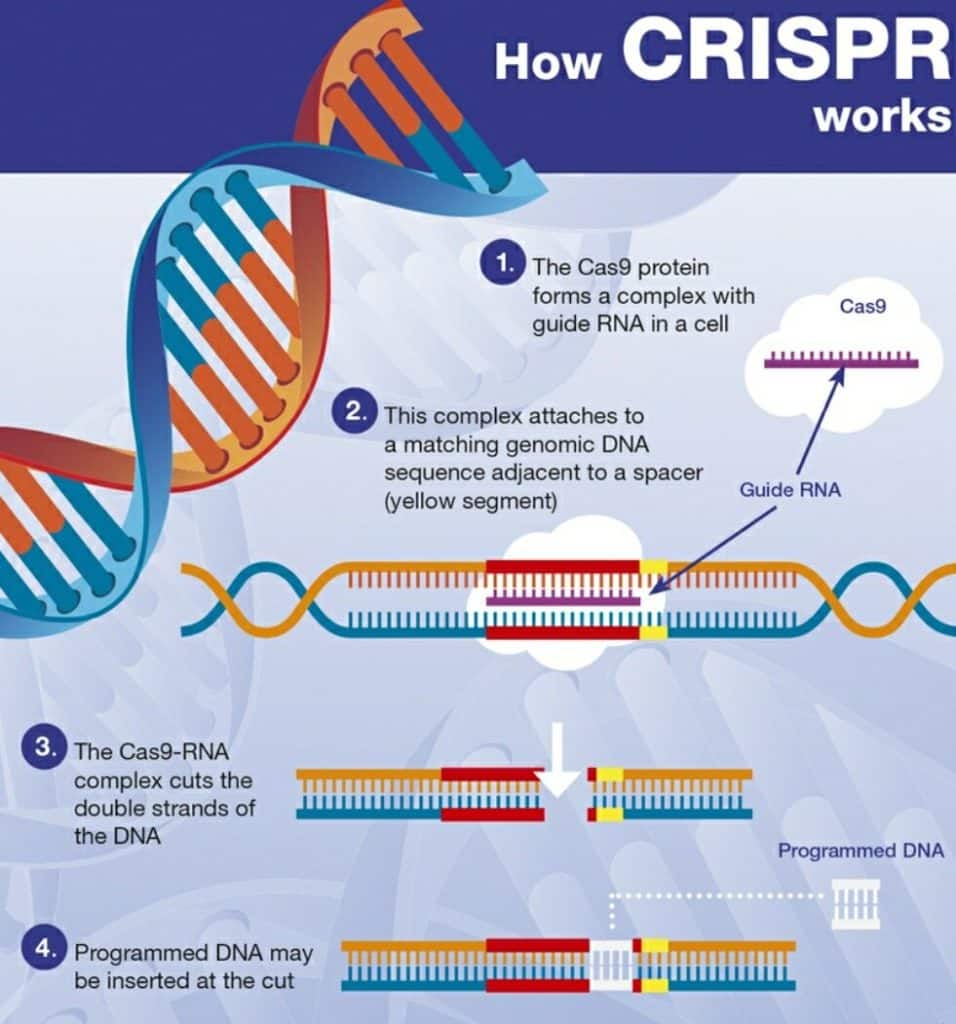
- The CRISPR-Cas9 system consists of two key molecules that introduce a change into the DNA. These are:
- an enzyme called Cas9. This acts as a pair of ‘molecular scissors’ that can cut the two strands of DNA at a specific location in the genome so that bits of DNA can then be added or removed.
- a piece of RNA called guide RNA (gRNA). This consists of a small piece of pre-designed RNA sequence (about 20 bases long) located within a longer RNA scaffold. The scaffold part binds to DNA and the pre-designed sequence ‘guides’ Cas9 to the right part of the genome. This makes sure that the Cas9 enzyme cuts at the right point in the genome.
- The guide RNA is designed to find and bind to a specific sequence in the DNA. The guide RNA has RNA bases that are complementary to those of the target DNA sequence in the genome. This means that, at least in theory, the guide RNA will only bind to the target sequence and no other regions of the genome.
- The Cas9 follows the guide RNA to the same location in the DNA sequence and makes a cut across both strands of the DNA.
- At this stage, the cell recognizes that the DNA is damaged and tries to repair it.
- Scientists can use DNA repair machinery to introduce changes to one or more genes in the genome of a cell of interest.
Base Editing
- Bases are the language of life. The four types of base – adenine (A), cytosine (C), guanine (G) and thymine (T) – are the building blocks of our genetic code.
- Just as letters in the alphabet spell out words that carry meaning, the billions of bases in our Deoxyribonucleic Acid (DNA) spell out the instruction manual for our body.
- A mis-arrangement in the sequence of bases may cause cancer.
- Base editing allows scientists to zoom to a precise part of the genetic code and then alter the molecular structure of just one base, converting it into another and changing the genetic instructions.
- The large team of doctors and scientists used this tool to engineer a new type of T-cell that was capable of hunting down and killing cancerous T-cells.
- Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology is one of the most popular approaches that allows the genes to be altered, thereby, fixing the errors.
- This method has been further improvised to be able to directly change certain bases such as a C can be changed into a G and T into an A.
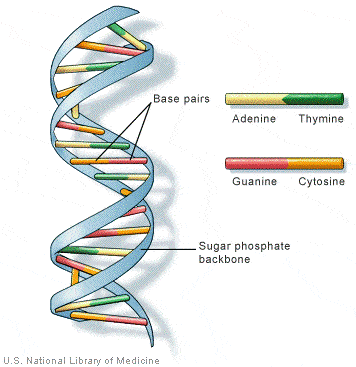
Chimeric Antigen Receptor T-cell (CAR-T) therapy
- It is a way to get immune cells called T cells (a type of white blood cell) to fight cancer by editing them in the lab so they can find and destroy cancer cells.
- T cells are taken from the patient’s blood and are changed in the lab by adding a gene for a man-made receptor (called CAR).
- This helps them better identify specific cancer cell antigens. The CAR T cells are then given back to the patient.
- It is also sometimes talked about as a type of cell-based gene editing because it involves altering the genes inside T cells to help them attack cancer.
- In order to promote and support the development of CAR-T cell technology, Biotechnology Industry Research Assistance Council (BIRAC) and the Department of Biotechnology (DBT) have taken initiatives in the last 2 years.
- Development of CAR-T cell technology for diseases including acute lymphocytic leukemia, multiple myeloma, glioblastoma, hepatocellular carcinoma, and type-2 diabetes is supported through DBT.
- Though this technology has a remarkable therapeutic potential for cancer patients, at present this technology is not available in India.
- Each patient’s CAR-T cell therapy costs 3-4 crore (INR). The challenge, therefore, is to develop this technology in a cost-effective manner and make it available to the patients.
- The manufacturing complexity is a major reason for the therapy cost.
Orphan Drug
A biological product or medicine that is intended to treat diseases so rare that sponsors are reluctant to develop them under usual marketing conditions.
- In 1983, the US government passed the Orphan Drugs Act to stimulate research in the treatment of diseases that have been largely ignored by the pharmaceutical industry. Similar laws have been enacted in Japan, Australia, and the European Union. All these laws offer incentives such as shorter clinical trials, extended exclusivity, tax breaks, and high rates of regulatory success. They have made it commercially attractive for pharmaceutical companies to invest in the research and development (R&D) required to find a cure for these diseases. India does not have a nationwide Orphan Drug policy.
- In 2016 Karnataka became the first state to release a Rare Diseases and Orphan Drugs Policy. It recommended the implementation of preventive and carrier testing as a means of reducing morbidity and mortality. Given that over 80% of rare diseases have a genetic basis, it suggested the use of genetic testing to accelerate the identification of the critical genes involved in rare diseases.
- The court made many suggestions to the government. It pointed to the corporate social responsibility (CSR) provisions under the Companies Act, 2013, and confirmed that the act of sponsoring the treatment of rare diseases would qualify as a CSR activity.
Bioprospecting
- It is the process of discovery and commercialization of new products based on biological resources. Despite indigenous knowledge being intuitively helpful, bioprospecting has only recently begun to incorporate such knowledge in focusing screening efforts for bioactive compounds.
- Biopiracy is the term used to refer to the use of bio-resources by multinational companies and other organizations without proper authorization from the countries and people concerned without compensatory payment.
- Biomining is a technique of extracting metals from ores and other solid materials typically using prokaryotes or fungi. These organisms secrete different organic compounds that chelate metals from the environment and bring it back to the cell where they are typically used to coordinate electrons.
- The people of India in a variety of ways have used neem, since time immemorial. Indians have shared the knowledge of the properties of the neem with the entire world. Pirating this knowledge, the USDA and an American MNC W.R. Grace in the early 90s sought a patent (No. 0426257 B) from the European Patent Office (EPO) on the “method for controlling on plants by the aid of hydrophobic extracted neem oil.” The patenting of the fungicidal properties of Neem was an example of biopiracy.
Biomaterials
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one.
Experts say a number of tissues can potentially be retrieved and stored for use. The Transplantation of Human Organs (Amendment) Act, 2011, includes the component of tissue donation and registration of tissue banks as well.
Biomaterials that can be potentially retrieved and stored –
- Skin: It is used as a biological dressing, in cases of major burns. It helps prevent infections and does not need to be changed every day – it can be kept for a couple of weeks, giving the patient time to recover.
- Bones: Bones from limbs can be stored and used to replace parts that are damaged or diseased. Bone grafts from banks act as scaffolds for support. They could be used in cases of trauma where there is bone loss, in sports injuries, and in cancer cases where parts of the bone and joint cartilage die. The upper end of the shin bone, the lower end of the thigh bone, and the head of the thigh bone can be retrieved for use.
- Ligaments and tendons: These can be used in cases of sports injuries involving multiple ligaments. In some cases, it is difficult to use the patient’s own. The Achilles tendon (ankle), the Peroneal tendon (leg to ankle), the Patellar tendon (front of the knee), and the Meniscus (a shock absorber between the thigh bone and leg bone) can be procured for storage.
- Bone products: Bone powder is made by crushing bones, generally those that would otherwise be disposed of – such as those parts replaced during hip replacement surgeries. These are used to treat various kinds of defects – in dentistry, skeletal and joint reconstruction procedures.
- Amniotic membrane: This is the wall of the amniotic sac. When a baby is delivered, the sac ruptures. The sac can be used as a biological dressing for burns, bedsores, diabetic ulcers, and skin reactions to radiation.
- Heart valves: Heart valves can be retrieved and stored to be used in valve replacement procedures. The advantage with such valves is that the patient does not need blood thinners. They are also cheaper than artificial valves. However, they last about 15 years, and another procedure may be subsequently required. Even in cases where the heart can’t be used, the valve can be retrieved for storage. Usually, the aortic valve is procured.
- Corneas: Corneal transplants are used in cases when the cornea becomes opaque – due to injuries, infections, birth defects, or rarely after surgeries.
Escherichia Coli
- Escherichia coli, also known as E. coli, is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms.
- The E. coli genome was the first to be completely sequenced (in 1997). E. coli is the well-understood bacterium in the world and is an extremely important model organism in many fields of research, particularly molecular biology, genetics, and biochemistry.
- It is easy to grow under laboratory conditions, and research strains are very safe to work with. As with many bacteria, E. coli grows quickly, this allows many generations to be studied in a short time. In fact, under ideal conditions, E. coli cells can double in number after only 20 minutes.
Luciferase
Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence and is usually distinguished from a photoprotein.
- Luciferases are widely used in biotechnology, for microscopy, and as reporter genes, for many of the same applications as fluorescent proteins. However, unlike fluorescent proteins, luciferases do not require an external light source but do require the addition of luciferin, the consumable substrate.
- Luciferases can be produced in the lab through genetic engineering for a number of purposes. Luciferase genes can be synthesized and inserted into organisms or transfected into cells. Mice, silkworms, and potatoes are just a few of the organisms that have already been engineered to produce the protein.
- In the luciferase reaction, light is emitted when luciferase acts on the appropriate luciferin substrate. Photon emission can be detected by light-sensitive apparatus such as a luminometer or modified optical microscopes.
Restriction Enzymes
- In order to generate recombinant DNA molecules, we not only require the vector and insert DNA but also a method to precisely cut these DNA molecules and then join them together (ligation).
- The foundations of rDNA technology were laid by the discovery of restriction enzymes. They are also called as ‘Molecular Scissors’.
- These enzymes exist in many bacteria where they function as a part of a defense mechanism called the Restriction-Modification System.
- A restriction enzyme that selectively recognizes a specific DNA sequence and digests any DNA fragment containing that sequence.
- The term restriction is derived from the ability of these enzymes to restrict the propagation of foreign DNA (e.g. Viral/phage DNA) in a bacterium.
- The Restriction-Modification enzyme system within a given bacterium protects its DNA from digestion by methylation but can digest foreign DNA which is not protected by similar methylation.
- Different species of bacteria contain their own sets of restriction endonucleases and corresponding methylases.
- Three main classes of restriction endonucleases- type I, type II, and type III are present, of which, only type II restriction enzymes are used in rDNA technology.
PHOTODYNAMIC THERAPY
- Photodynamic therapy uses a photosensitive drug that becomes active under the action of light and converts molecular oxygen into reactive oxygen species that kill cancer cells.
- It uses special drugs, called photosensitizing agents, along with light to kill cancer cells. Depending on the part of the body being treated, the photosensitizing agent is either put into the bloodstream through a vein or put on the skin. Over a certain amount of time, the drug is absorbed by the cancer cells. Then light is applied to the area to be treated. The light causes the drug to react with oxygen, which forms a chemical that kills the cells. PDT might also help by destroying the blood vessels that feed the cancer cells and by alerting the immune system to attack cancer.
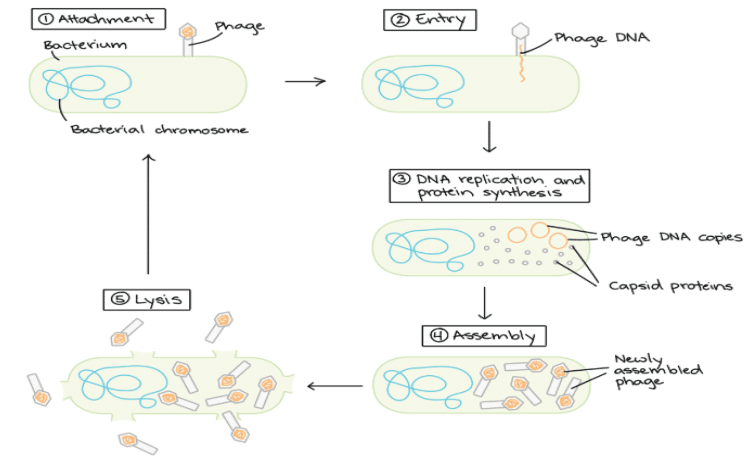
Bacteriophage
- Bacteriophages are viruses that infect bacterial cells by injecting their DNA into them and consequently take over the machinery of the bacterial cells to multiply themselves.
- The injected DNA hence is selectively replicated and expressed in the host bacterial cell resulting in a number of phages that eventually extrude out of the cell (lytic pathway) and infect neighboring cells.
- Some phages can only reproduce via a lytic lifecycle, in which they burst and kill their host cells.
- This ability to transfer DNA from the phage genome to specific bacterial hosts during the process of viral infection gave scientists the idea that specifically designed phage-based vectors would be useful tools for gene cloning experiments.
- Two phages that have been extensively modified for the development of cloning vectors are lambda (ë) and M13 phages.
CHARGE SYNDROME
- CHARGE syndrome is a rare birth defect that affects approximately 1 in 20,000 people around the world. CHARGE syndrome causes multiple life-threatening problems in a newborn, such as facial bone and nerve defects that cause breathing and swallowing difficulties, deafness and blindness, heart defects, genital problems and growth retardation.
- The children may survive and go on to live with these deficiencies if the heart and bone defects are corrected with multiple surgeries, but those without access to such support usually do not survive past their first year.
- CHARGE syndrome is a result of defective embryonic development. Two-thirds of the patients with CHARGE syndrome have a sporadic mutation in the gene called CHD7.
BIONICS
- Bionics is not a specialized science. It refers to the use of principles of biology to engineering. Now it is used more to describe a method to engineer organs that can replace diseased or non-functional organs in human body.
- Bionics is different from bioengineering (or Biotechnology), which is the use of living things to perform industrial tasks like use of microbes to remove waste etc.
Autophagy
- The word autophagy is derived from Greek words “auto” meaning self and “phagy” meaning eating. Autophagy is a normal physiological process in the body that deals with destruction of cells in the body.
- It maintains homeostasis or normal functioning by protein degradation and turnover of the destroyed cell organelles for new cell formation.
- During cellular stress the process of Autophagy is upscaled and increased. Cellular stress is caused when there is deprivation of nutrients and/or growth factors.
- Thus Autophagy may provide an alternate source of intracellular building blocks and substrates that may generate energy to enable continuous cell survival.
- Autophagy also kills the cells under certain conditions. These are form of programmed cell death (PCD) and are called autophagic cell death. Programmed cell death is commonly termed apoptosis.
- Autophagy is termed a nonapoptotic programmed cell death with different pathways and mediators from apoptosis.
- Autophagy mainly maintains a balance between the manufacture of cellular components and breakdown of damaged or unnecessary organelles and other cellular constituents.
- Thanks to Ohsumi and others following in his footsteps, we now know that autophagy controls important physiological functions where cellular components need to be degraded and recycled.
- Autophagy can rapidly provide fuel for energy and building blocks for the renewal of cellular components and is therefore essential for the cellular response to starvation and other types of stress.
- After infection, autophagy can eliminate invading intracellular bacteria and viruses. Autophagy contributes to embryo development and cell differentiation.
- Cells also use autophagy to eliminate damaged proteins and organelles, a quality control mechanism that is critical for counteracting the negative consequences of aging.
- Disrupted autophagy has been linked to Parkinson’s disease, type 2 diabetes, and other disorders that appear in the elderly. Mutations in autophagy genes can cause genetic disease. Disturbances in the autophagic machinery have also been linked to cancer. Intense research is now ongoing to develop drugs that can target autophagy in various diseases.
- Autophagy has been known for over 50 years but its fundamental importance in physiology and medicine was only recognized after Yoshinori Ohsumi’s paradigm-shifting research in the 1990s. For his discoveries, he is awarded the 2016 Nobel Prize in physiology or medicine.
TRIPLE DRUG THERAPY
The World Health Organization (WHO) is recommending an alternative three-drug treatment to accelerate the global elimination of lymphatic filariasis – a disabling and disfiguring neglected tropical disease.
- The treatment, known as IDA, involves a combination of ivermectin, diethylcarbamazine citrate, and albendazole. It is being recommended annually in settings where its use is expected to have the greatest impact.
- Lymphatic filariasis is caused by infection with parasitic worms living in the lymphatic system. The larval stages of the parasite (microfilaria) circulate in the blood and are transmitted from person to person by mosquitoes.
- The manifestation of the disease after infection takes time and can result in an altered lymphatic system, causing abnormal enlargement of body parts, and leading to severe disability and social stigmatization of those affected.
- The parasites are transmitted by four main types of mosquitoes: Culex, Mansonia, Anopheles, and Aedes.
- A day-long National Symposium on the theme ‘United to Eliminate Lymphatic Filariasis’ was inaugurated by Union Minister for Health and Family Welfare.
- The Union Minister also signed the ‘Call to Action to eliminate Lymphatic Filariasis by 2021’.
- India is set to scale-up the use of Triple Drug Therapy (IDA) in a phased manner starting from November 2019.

amazing content! a big thankYOU